Evaporator
Table of Contents
What is an Evaporator?
Evaporators are a type of heat exchanger which changes the phase of a refrigerant from liquid to gas. Pressure will be reduced in the system when the liquid refrigerant arrives at the evaporator. This refrigerant will then release heat making itself cooler than the air from the fan around it. Now the refrigerant absorbs heat from the warm air and will reach its low boiling point rapidly, then vaporizes, absorbing the maximum amount of heat, [1]. Below is an image of an industrial evaporator system.
| Industrial Evaporators [2] |
|---|
General Outline of How Evaporators Work
The liquid solution is fed into the evaporator and passes across the heat source. The applied heat vapourizes the liquid water. The vapour is moved out of the system and is condensed. The concentrated solution is moved into a second evaporator stage or is removed, depending on the type of evaporator.
Evaporators generally have four different sections, the heating section, concentrating/separating section, condensing section, and the pump/vacuum section. The heating section heats the solution, contained within the heating surface. The most common medium consists of parallel tubes, but other mediums may have plates or coils, which are typically made from copper or aluminum. The concentrating/separating section separates the vapour from the solution, which concentrates the solution. The condenser condenses the vapour. The vacuum/pump provides pressure to increase circulation [3].
Types of Evaporators
Natural Circulation Evaporator
Natural circulation evaporators leverage the natural circulation of the product caused by the difference in density caused by heating. For example, in an evaporator using enclosures such as tubing, the bubbles caused by boiling will rise and cause circulation. The bubbles also facilitate the separation of the liquid and the vapour at the top of the heating enclosure. Evaporation is then dependent on the temperature difference between the vapour and solution [4].
| Natural Circulation Evaporator Illustration [5] |
|---|
Forced Circulation Evaporator
Natural circulation evaporators can lead to problems such as drying out and fouling. To avoid these problems, a pump can be installed. Forced circulation uses a hydrostatic head to prevent boiling at the heating element surface. The pump decreases the amount of bubbles formed and results in less fouling at the heating elements surface [6].
| Natural and Forced Circulation Evaporator Illustration [7] |
|---|
Falling Film Evaporator
The heating medium of this type of evaporator is typically made of long (4-8m) tubes with the source of heat being steam jackets. Equal distribution of the solution is important to maximize the efficiency of this type of evaporator. The solution is fed into the top of the tubes, where it gains velocity as it flows downward. The speed of the solution flowing along the heating surface is proportional to the amount of vapour that is produced. This evaporator is usually used with viscous solutions [8].
Rising Film Evaporator
This type of evaporator is very similar to the falling film evaporator, but the solution is fed from the bottom and travels upwards, rather than downwards. The vapour produced moves upwards as well, which increases the efficiency of the evaporator. The main disadvantage comes from the heating surface being prone to fouling. That is why the solutions used in this type of evaporator are clear and don’t have high levels of salinity. Note that this type of evaporator is not as common as the falling film evaporator [9].
Climbing and Falling-Film Plate Evaporator
Climbing and falling-film plate evaporators main advantage is their large surface area. As shown in the image below, there are parallel plates that are supported. During evaporation, vapour flows through the channels between the plates. The steam climbs/falls parallel to the concentrated liquid. So, if the liquid is falling, the vapour is climbing. The concentrate and vapour are fed into the separation stage, where the vapour is further sent to a condenser. This type of plate evaporator is found in the dairy and fermentation industries. The type of evaporator cannot be used with viscous solutions or solutions with solids [10].
| Illustration of Climbing and Falling-Film Plate Evaporator [11] |
|---|
Agitated Thin Film Evaporators
Agitated thin film evaporators use indirect heat transfer and agitation to separate volatile components from less volatile components of the solution. Vacuum conditions are used in the separation stage to maximize the temperature difference and ensure appropriate temperatures are maintained. Agitated thin film evaporators are generally used for sensitive solutions [12].
| Illustration of an Agitated Thin Film Evaporator [13] |
|---|
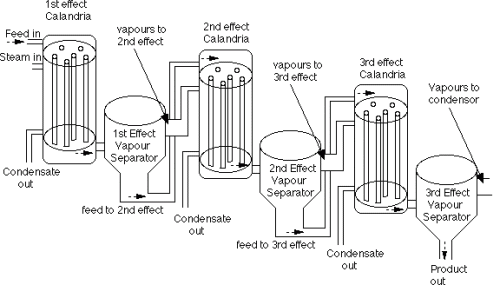
Multiple-Effect Evaporators
Multiple-effect evaporators are evaporators combined in series. The series combination is used to save heat, which in effect reduces the amount of energy used. Increasing the number of evaporators reduces the amount of energy required [14].
| Illustration of Multiple-Effect Evaporator [14] |
|---|
Applications
Like condensers, evaporators are heat exchange units. Examples of daily uses for evaporators are in air conditioning and refrigerators. Some of the most important applications of evaporators are in the food industry. Food or beverages that need to be preserved or need to have a certain consistency, like coffee, go through an evaporation step during processing. Another important application is to reduce moisture, which is critical in the pharmaceutical industry [15].
Contributors:
| User | Last Update |
|---|---|
| Former user (Deleted) | 1423 days ago |
| Former user (Deleted) | 1425 days ago |
| Former user (Deleted) | 1432 days ago |
Faculty Advisor: Sarah Meunier