Table of Contents
Table of Contents maxLevel 4
What is Copper?
Copper is a natural element that can be found on the periodic table (symbolized by Cu) and is widely used in our lives. It is reddish-brown in colour, is an extremely ductile metal [1], and has excellent corrosion and hygienic properties [2]. It has a melting point of 1083 °C and since it is a metal, it is a good conductor of electricity and heat [1]. Specifically, pure copper has the best electrical and thermal conductivity of any commercial metal [2]. Copper is commercially produced primarily by smelting or leaching [1] and is able to be hardened by working [2]. The majority of the copper produced in the world is used by the electrical industries, and the remainder is combined with other metals to form alloys [1].
| Pure copper [ | 32] |
Copper Alloys
Copper’s properties can be improved to fit the needs of many industrial applications by alloying, which is making a solid material out of two or more different metals. Copper alloys are grouped into families based on their metal composition, which provide variations of properties. Copper forms alloys more easily than most metals and has a wider range of alloying elements.
- Brass
- Bronze
- Gunmetal
- Copper nickel
- Beryllium copper [23]
Brass
Brass is an alloy made of copper and zinc. Basic modern brass is usually 67% copper and 33% zinc, but the compositions of the elements may vary (55% to 95% for copper and 5% to 45% for zinc). This metal alloy is typically bright gold in colour, but can be reddish-gold or silvery-white as well. If there is a higher percentage of copper, there will be a rosier tone, whereas more zinc causes it to look silver. Brass has low friction when in contact with other materials, is a soft metal, is a good conductor of heat, resistant to corrosion, easy to cast, and is not ferromagnetic [4]. The malleability of brass depends on the zinc content; alloys containing more than 45% zinc are not workable [5]. Brass has a low melting point of 900-1000°C, depending on its composition and even possesses some antimicrobial qualities due to its high copper content. Aside from containing copper and zinc, lead may be added to increase its machineability [6].
| Objects made from brass [ |
| 6] |
Bronze
Bronze is an alloy traditionally made of copper and tin, but some modern bronzes have no tin and are instead composed of copper and other metals such as aluminum, manganese, or even zinc. As a result of being alloyed with tin, bronze is harder that pure copper. Additionally, bronze is more fusible (readily melted) and easier to cast. Aluminum bronzes, which contain up to 16% aluminum and small portions of other metals such as iron or nickel, are particularly strong and corrosion resistant [107]. Typically, bronze is a reddish-brown or gold colour and is brittle. It exhibits low friction when in contact with other substances (such as other metals), readily conducts heat and electricity, and has a melting point ranging from 950-1050°C, depending on its composition.
| Bronze for precision machining [ |
| 8] |
Corrosion Resistance
Bronze oxidizes in the air because of its high copper content, which may give bronze a mottled patina. Mottled refers to have spots or smear of color, and a patina is a green or brown film on the surface of the bronze object. This oxidation prevents the bronze from corroding, particularly in saltwater environments.
However, a process called ‘bronze disease’ can occur if chlorine compounds can react with the bronze. In this process, corrosion (the oxidization) causes more corrosion to occur, slowly destroying the alloy [69].
| Bronze patina formation [ |
| 10] |
| Close up of item with bronze disease [ |
| 11] |
Gunmetal
Gunmetal is a variety of bronze and is an alloy typically consisting of 88% copper, 10% tin, and 2% zinc [1612]. However, the term gunmetal is applied to the family of copper alloys that contain between 2-11% tin and 1-10% zinc [1713]. It is able to withstand atmosphere, steam, and seawater corrosion [1612]. Gunmetal is easily casted and has good strength [1712].
| Gunmetal bushing (also known as red brass) [ |
| 14] |
Copper Nickel
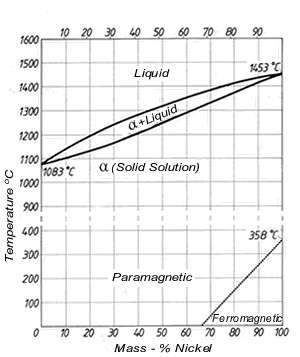
Copper nickel is a metal alloy consisting of copper and nickel. The addition of nickel to copper increases the strength and resistance to corrosion while remaining ductile [2115]. The minimum service temperature for copper nickel is -273.15°C and the maximum service temperature is 206.85°C. Regarding conductivity, copper nickel has a low temperature co-efficient of electrical resistance, meaning its resistance decreases with the increase of temperature. As a result, its performance will be consistent regardless of temperature [2216].
| Copper nickel | barsrods [ | 2317] | Figure 12:
| Equilibrium diagram of copper nickel alloys [ |
| 18] |
|
|---|
Corrosion Resistance
Copper nickel alloys rely on a protective surface film to maintain its corrosion resistance. This passive protective layer is formed as a result of a reaction with seawater. Initially, the layer develops quickly in a short period of time but continues to develop overtime. Additionally, to ensure that the protective layer forms correctly, it is important to allow the copper nickel to be initially exposed to appropriate seawater conditions [2519].
| Passive protective layer forming [ | 2620] |
Copper nickel alloys are not susceptible to pitting, crevice corrosion, or stress corrosion cracking induced by chlorine. However, sulfides may change the nature of the protective film, possibly leading to pitting and higher corrosion rates in these circumstances. Therefore, conditions in which the material is exposed to sulfides should be avoided [2520].
Beryllium Copper
Beryllium copper is one of the highest strength copper alloys available today. Commercial grades of beryllium copper consist of 0.4-2% beryllium, and the small ratio between beryllium and copper create a family of alloys with strength nearly as high as alloy steel. This alloy yields great results when subjected to precipitation-hardening treatments, have good thermal conductivity [3021], and is nonmagnetic. Beryllium copper’s hardness is extremely versatile, as it can be either softened or hardened as required by applying different heat treatment processes. Like other copper alloys, it is highly resistant to corrosion [3122].
| Beryllium copper rods [ |
| 23] |
Advantages and Disadvantages of Copper and Copper Alloys
Advantages | Disadvantages |
|---|---|
|
|
Applications of Copper and Copper Alloys
Applications of Brass
Brass pipes are good in plumbing environments as well as oil, gas, steam applications, and for hot water distribution lines. When transporting hot water, brass does not change the properties of the water since it doesn’t rust and tend to have a longer life. Additionally, the softness of brass makes for a tight seal [
27].
| Steam engine with brass pipes [ |
| 28] |
Applications of Bronze
Bronze may be casted or working into pipe fittings, pumps, gear, ship propellers, and turbine blades [
107]. Due to its saltwater resistance, bronze is useful for boat fittings and submerged marine parts. Additionally, bronze is often used for sculptures since they must resist degrading in an outside environment [
69].
| Bronze fittings [ |
| 29] |
| Vintage bronze pipes for a french horn [ |
| 30] | |
Applications of Gunmetal
Gunmetal is used for gears and bearings that may be subjected to heavy loads and low speeds. Since it has good corrosion qualities and is known to able to be manufactured in intricate, pressure tight castings, it is suitable for valves, pump parts, and steam fittings [
1612][
1713].
| Gunmetal gear [ |
| 31] |
| Gunmetal fittings [ |
| 32] | |
Applications of Copper Nickel
Since copper nickel allows have high resistance to seawater corrosion, they are often used for marine applications. Some of these include power generation involving marine renewable energy (wave and tidal devices) and the components of seawater systems [
2733]. Generally, the alloys that are chosen for sea water equipment contain between 10 to 30 percent nickel. Other than being highly resistant to corrosion, copper nickel pipes are resistant to various sea organisms such as barnacles, making it reliable to be used for seawater desalination, firefighting systems, and other water systems [
2836].
| Marine power generation plant [ |
| 33] |
| Seawater inlet pipe [ |
| 33] |
|
|---|
In ship systems, seawater is used for various purposes such as cooling, heating, or a source of freshwater. Therefore, it is essential to use pipes to transport this seawater throughout the ship. By using copper nickel pipes specifically, seawater can be transported without fear of premature pipe failure [
2935].
| Seawater piping system [ |
| 33] |
| Seawater system components [ |
| 33] | |
|
|---|
Applications of Beryllium Copper
Beryllium copper alloys are usually used in very specific situations and are often tailor-made. Some of these applications include oilfield tools, aerospace landing gears, robotic welding, and mold making [
3021].
| Beryllium copper bushings and bearings in aircraft landing gears to withstand high compressive forces [ |
| 36] |
Pipe Sizing
| Copper pipe and tubing sizing chart [37] |
Contributors:
| Contributors Summary | ||||
|---|---|---|---|---|
|
Faculty Advisor: Mary Robinson








:max_bytes(150000):strip_icc()/metals--copper--bars-of-copper-for-further-industrial-proces-sing-----121849145-5945c5385f9b58d58af7f6b5.jpg)










