Table of Contents
| Table of Contents |
|---|
What are Fuel Cells?
A fuel cell is an electrochemical device that generates electricity through a chemical reaction. Fuels cells are used in various applications such as powering homes, stores, and hospitals [2]. Fuel cell systems are clean, efficient, and reliable as they continue to produce electricity as long as a fuel source is available compared to batteries which need to be recharged often.
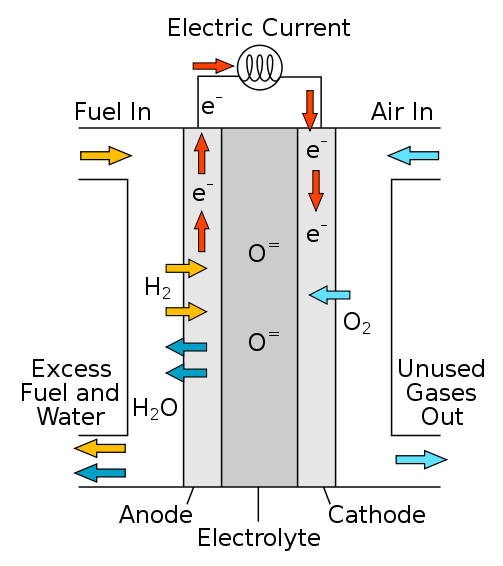
Fuel cells are composed of a cathode, electrolyte membrane, and anode. When hydrogen is the fuel, a fuel cell works when hydrogen passes through the anode and a catalyst splits these hydrogen molecules into electrons and protons [2]. The protons of this molecule pass through the electrolyte membrane and the electrons are forced through a circuit which generates an electric current [2]. At the cathode, oxygen passes through where the protons, electrons, and oxygen combine to produce water. This process results with the products being electricity, water, and heat.
| Figure 1: Hydrogen Fuel Cell [1] |
Types of Fuel Cells
Fuels cells are classified by the electrolyte they utilize. This in turn affects the electrochemical reactions taking place, the catalysts used, the fuel needed, and much more. These also affect the applications in which these cells can be used for.
Polymer Electrolyte Membrane Fuel Cells (PEMFCs)
Also known as proton exchange membrane fuel cells, this fuel cell delivers a high power density and has a low volume and weight compared to other cells [3]. A solid polymer electrolyte and porous carbon electrons with a platinum catalyst is used within the cell and require hydrogen, oxygen and water to operate. PEMFCs can be used to provide power to cars and buses [3].
Direct Methanol Fuel Cells (DMFCs)
DMFCs are powered by pure methanol which is mixed with water and then fed to the anode. The fuel storage issues are not much of an issue as it has a higher energy density than hydrogen and it is in a liquid form [3]. DMFCs can be used to provide power to cell phones or laptops.
Alkaline Fuel Cells (AFCs)
Alkaline Fuel Cells were one of the first fuel cell technologies developed [3]. They use potassium hydroxide in water as the electrolyte and metals as a catalyst at the anode and cathode. Recently, they have been modified to use an alkaline membrane as the electrolyte.
Solid Oxide Fuel Cells (SOFCs)
Solid Oxide Fuel Cells use a ceramic compound as the electrolyte [3]. SOFCs operate at high temperature which eliminates the need for metal catalysts and reforms the fuels internally. SOFCs can use natural gas, biogas, and gases made from coal [3].
Reversible Fuel Cells (RFCs)
Reversible fuel cells draw from the same concept as PEMFCs however they can use electricity from renewable sources such as solar power or wind power and split water into oxygen and hydrogen fuel through electrolysis. During times of high power production (e.g. a very windy day) these cells can store the excess energy in the form of hydrogen. This in turn helps overcome the intermittent nature of these renewable resources.
Gold Nanoparticle Catalysts for Fuel Cell Reactions
Nanostructured catalysts are valuable in fuel cells as they improve the energy density, power density, operation reliability of the fuel cell while reducing expenses. Along with high surface area, they have good conductivity, low polarization and unique chemical properties.
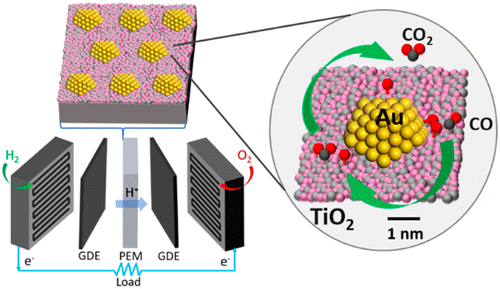
Gold nanoparticles (AuNPs) are being extensively used as a catalyst material in fuel cells. Gold nanoparticle catalysts have a large advantage over other catalysts such as platinum, due to cost. For certain fuel cells such as DMFCs, AuNP catalysts have shown higher catalytic activity because of their surface structure. The high surface area of the nanoparticle also allows for reactant molecules to easily attach. Gold catalysts have also shown to exhibit high performance in the selective oxidation of CO which is key in the development of PEMFCs and AFCs.
| Figure 2: Gold Nanoparticle Catalyst in PEMFC [4] |
References
Contributors:
| Contributors Summary | ||||
|---|---|---|---|---|
|
Faculty Advisor: Ahmad Ghavami